Introduction: Acute myeloid leukemia/myelodysplasia (AML/MDS) may harbor pathogenic mutations not detected by karyotyping and fluorescence in-situ hybridization; hence, next-generation sequencing (NGS) is a value-added modality that affords high-quality stratification for risk and therapy. If, however, NGS is not clinically indicated and, when performed, adds data of unknown significance, such data may promote futile avenues of investigation, patient emotional distress, and increased cost. We propose objective criteria, using evidence-based indications and our experience, to approve or cancel AML/MDS NGS testing, with the goals of: [1] maximizing actionable variant detection and [2] minimizing unnecessary testing. These criteria specifically include [a] clinical suspicion of new, progressive, or relapsed AML/MDS, [b] the presence of non-myeloid disease, [c] donor chimerism status after transplant, [d] whether or not circulating disease is present, and [e] whether or not the sample is blood or marrow. These criteria are further summarized in Table 1.
Methods: Consecutive NGS orders were retrospectively collected from the molecular diagnostics laboratory and categorized as performed or cancelled up-front. The medical record for each patient was reviewed for specimen type, clinical indication, pathologic diagnosis, and molecular findings. These orders were categorized and analyzed according to our proposed criteria.
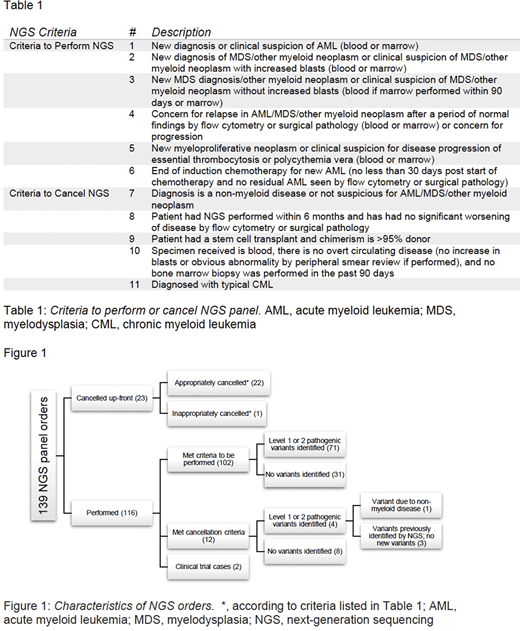
Results: AML/MDS NGS consecutive orders (n=139) performed from September to December 2018 identified 102 appropriately ordered tests and 12 orders that were carried through testing but would have been cancelled according to the proposed criteria. Four of these 12 (33%) tests identified pathogenic variants, compared with 71 of the 102 (70%) approved tests. These four inappropriately ordered tests included [a] 3 patients in whom there was no AML/MDS progression, prior known variants were re-identified, and no new variant was detected; and [b] 1 patient with a variant attributable to their non-myeloid neoplasm. The remaining 25 tests included 2 performed for a clinical trial and 23 that were cancelled up-front (22 appropriately) based on our proposed criteria. This suggests that approximately 25% (34/139, 24.5%) of AML/MDS NGS testing can be safely and appropriately cancelled using objective criteria. If the 12 tests had been cancelled up-front according to the criteria, approximately $9,360 could have been saved based on current Centers for Medicare and Medicaid Services (CMS) reimbursement. These results are summarized in Figure 1.
Discussion: Overall, the cancellation criteria excluded cases that were non-beneficial clinically. In the cases that had NGS performed but met our cancellation criteria, no significant variants were identified, supporting our recommendation. Additionally, the cost and time of reporting NGS studies is significant, and many laboratories can only perform a limited number of NGS tests per week. Thus, our criteria for performing or cancelling the AML/MDS NGS test may allow for more precise and cost-efficient myeloid neoplasm evaluations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal